
A significant amount of work and investment on behalf of providers has gone into attesting to the various stages of meaningful use over the past few years. The hope is to achieve better clinical outcomes, improved population outcomes, increased transparency and efficiency, empowered individuals and more robust research data on health systems. In this blog series, we will discuss different areas of MU3 in hopes that you will be more informed on the resources required to meet each measure. Much of this process requires dedicated attention of experienced staff and you will need to determine if you have the adequate resources to attest to MU3 by the January 2018 deadline.
As MEDITECH releases application codes and top offs throughout 2017, you must consider how your organization will approach hardware requirements, testing interfaces, data extract and reporting. Once you receive the initial application code required for MU3 reporting from MEDITECH, we advise you to update quickly to allow for as much time as possible to test interfaces and establish new workflows.
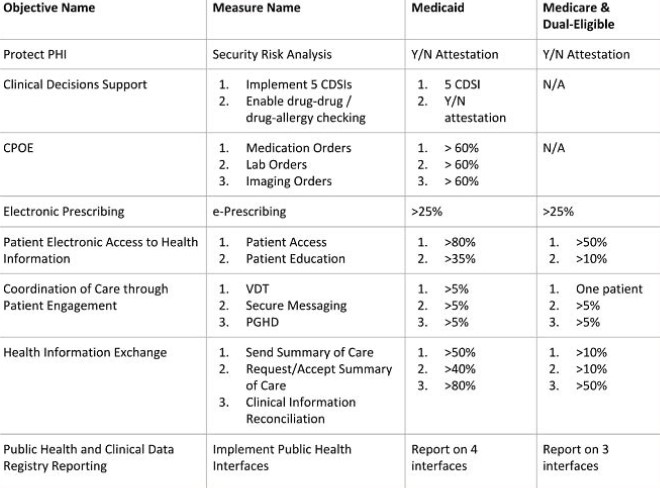
It’s important to remember, you must report on clinical quality measures (eCQMs) for the full calendar year in order to successfully attest to MU3. Recently, CMS announced an extension of the 2016 reporting deadline and intends to modify 2017 requirements for eCQM data. You can read more about this extension here. For Medicare and dual-eligible hospitals, there are new rule changes to take into account; you no longer have to report on CPOE and CDSi objectives and thresholds have been lowered. An overview of objectives and measures for Medicaid hospitals and Medicare and dual-eligible hospitals is presented in the chart below:
With the help of MEDITECH, we’ve outlined some key points within each objective and what is and isn’t required in terms of applications, staffing and third party vendors:
Protect Patient Health Information
All participants must complete or review one security risk analysis measure each calendar year. For Stage 3, the implementation of appropriate technical, administrative and physical safeguards is required. You can use a third party vendor to conduct this analysis or review though it’s not required. You’ll need a technical staff to review security and implement corrections.
Clinical Decision Support
Medicaid Hospitals must implement 5 Clinical Decision Support Interventions (CDSi) and enable drug-drug and/or drug-allergy interaction checking. Medicare and dual-eligible hospitals are no longer required to report this object and no changes have been made to this objectives measures. To implement 5 CDSi’s, you can utilize any application and you will need a clinical staff to determine interventions and a technical staff to create notifications.
When enabling drug interaction and drug-allergy checks, note that you must implement functionality for an entire EHR reporting period. You will then use the Physician Ordering/PHA application and you will need a clinical staff to build and train your staff.
Computerized Provider Order Entry (CPOE)
This objective is only required for EHs/CAHs attesting to Medicaid. Using the following applications, POM/OM, PHA, LAB and ITS/RAD, you must report more than 60% for each type of order. It’s important to note there was a 30% increase in the lab and imaging measures. You will need a dedicated clinical staff for the build and staff training.
ePrescribing (eRX)
All participants must electronically transmit more than 25% of discharge medications (an increase of 10%) using the RXM application. It’s required that Dr. First is used as the third party vendor and this also requires an update to the MEDITECH interface. You will need a clinical staff for building and training as well as a technical staff to test and troubleshoot the interface.
Patient Electronic Access to Health Information
Measure 1 requires that patient health information must be made available to the patient within 36 hours of its availability to the provider. For Medicare and dual-eligible hospitals, 50% of patients must have access to their health information via view, download, transmit and API applications and for Medicaid hospitals, 80% of patients must have access. Any third party application selected by a patient to request their health information must meet the specifications of MEDITECH’s API. You are required to use the MT Portal, CCD with direct and API as well as HISP for your third party vendor. You will need a technical staff for interface, direct and API testing and troubleshooting and a clinical staff for build.
Measure 2 requires that 10% (Medicare and dual-eligible hospitals) and 35% (Medicaid hospitals) patients receive education suggested by the CEHRT based on a problem list or medication list and it must be provided electronically. The MT Portal and CCD with Direct is required and you will be using the NUR/PCS and EPS/EMR applications. There is an option to use a third party vendor for content but it’s not required. Note that a non-MEDITECH portal will require an interface to send the education. You will need a clinical staff for building and training.
Coordination of Care through Patient Engagement
Measure 1 requires that for Medicare and dual-eligible hospitals, at least 1 patient must view, download and transmit or access data via the API application and for Medicaid hospitals, it’s more than 5% of patients. You must use the MT Portal, CCD with Direct and API and the patient selected application must meet your EHR’s technical specifications. You’ll need a technical staff for interface testing.
To comply with Measure 2, more than 5% of patients must receive a message from a provider. You must use the MT Portal and CCD with Direct within the PCM and PHM applications. A non-MEDITECH portal will require a third party solution. You will need a technical staff for interface testing and troubleshooting and a clinical staff for message composition.
For Measure 3, more than 5% of patients must have health data via a scanning solution or providers can receive a message from a patient with a link to an internet address where they have uploaded or documented their health information. You will use the SCA or PCM/PHM application and you will need to train your staff accordingly.
Health Information Exchange
Measure 1 requires more than 10% of patients in Medicare and dual-eligible hospitals and more than 50% in Medicaid hospitals have a continuity of care document (CCD) sent. A CCD interface is required and you can use designated clinical and administrative applications as well as HISP for Direct as your third party vendor. You will need a clinical staff to build the CCD and a technical staff to test and troubleshoot the interface, Direct or any other solution.
Measure 2 states that more than 10% of new patients in Medicare and dual-eligible hospitals and more than 40% in Medicaid hospitals have a CCD received and incorporated. You will need a CCD interface or Direct and you’ll use the EPS/EMR and DPT/ITS applications. Your third party vendor should be HISP for direct messaging and you’ll utilize a clinical staff for training and a technical staff for testing and troubleshooting.
Measure 3 requires more than 50% of new patients in Medicare and dual-eligible hospitals and more than 80% in Medicaid hospitals have clinical information such as a problem list, home medications and medication allergies reconciled via the RXM/AOM, EPS/EMR, PHA and PCM application. You will need third party vendor, IMO, for a problem list and FSV for allergies and home medications. A clinical staff to train clinicians is required.
Public Health Reporting
Medicare and dual-eligible hospitals must report on 3 of the 6 measures and Medicaid hospitals must on 4 measures or claim exclusions. Note that exclusions don’t count towards the total. New interfaces and updates to previously licensed interfaces (no additional cost) are available. You will need a clinical staff for data capture and mapping and a technical staff for interface testing and troubleshooting.
Additionally, AUR reporting does not require an interface. However, you will need a SQL report solution that generates monthly and will be uploaded to the National Healthcare Safety Network’s website. AUR also requires manual mapping with yearly updates.
If you are considering a partner, our MEDITECH team is READY certified and has extensive operator experience. Whether you need an assessment and a road map, staff augmentation, application support or technical assistance, our resources can fill in the gaps of your organization and we are happy to discuss how we can help you achieve attestation to MU3. To contact us, please email info@cerecore.net or call us at 855.276.9112.
For more detailed information, you can reference MEDITECH’s regulatory resources here.
Read more about our work with Cass Regional.
CereCore® provides IT services that make it easier for you to
CereCore® provides IT services that make it easier for you to
What happens when bold leadership meets groundbreaking technology?
Mater Private Network is one of Ireland’s leading private healthcare providers, known for delivering world-class services in medical and surgical care.
Despite the Federated Data Platform being an NHS England priority for years, it still feels like a solution in search of a problem. Not because the NHS is short on problems – if only – but because...
Let us know how we can support your initiatives and take some of the heavy lifting from healthcare IT.

© All Rights Reserved CereCore Terms of Service Notice at Collection Privacy Policy Do Not Sell My Personal Information Responsible Disclosure